GxPs for NIS and Observational Studies
Quick Reference Guide
- Is GCP applicable to Non-Interventional Studies (NIS)?
Is GPP the Equivalent for GCP for Observational Studies?
Which of the GxPs are Applicable to Non-Interventional Studies and Observational Studies?
Is GCP Applicable to Non-Interventional Studies?
The short answer is NO.
The longer, more detailed answer, starts with re-phrasing the question…”when designing, conducting, reporting and archiving non-interventional studies (NIS) do we need to comply with ICH GCP?”
ICH GCP = ICH E6, meaning it is guideline number 6 (of 20) in the ICH ‘Efficacy’ series. According to the ICH website: “The work carried out by ICH under the Efficacy heading is concerned with the design, conduct, safety and reporting of CLINICAL TRIALS. It also covers novel types of medicines derived from biotechnological processes and the use of pharmacogenetics/ pharmacogenomics techniques to produce better targeted medicines.”
THE FOCUS OF ICH GCP IS CLINICAL TRIALS, which is why there is reference to “investigational product (IP)”, the “investigator’s brochure (IB)” and research “subjects/trial subjects” who are the recipients of “investigational product(s)”. None of this applies to non-interventional studies, where we assess the effectiveness of routine treatments and clinical practice in patients.
ICH GCP is embedded in clinical trial legislation which is why it is legally enforceable for clinical trials (see EU example below). The same clinical trial legislation is not applicable to non-interventional studies (usually explicitly stated as such). Meaning? ICH GCP is not applicable to non-interventional studies from a legislative and regulatory compliance perspective.
You are free to follow the principle of ICH GCP (e.g., informed consent process and trial mster file content), but you are not legally obliged to do so.
Is GPP the Equivalent of GCP for Observational Studies?
The short answer is NO.
ICH GCP is a comprehensive guideline that has been embedded in, and enforced through, clinical trial legislation globally (see above). ISPE GPP is not embedded in, or enforced through, observational study legislation…anywhere. However, ISPE GPP is referenced in regulator guidance as a recommendation that shoud be “considered” (see examples in the table below).
| Regulator | Reference to GPP |
|---|---|
| BfArM | Section 2.7 of the BfArM/PEI Joint Recommendations of December 2019 |
| EMA | Section VIII.B.1 of GVP Module VIII |
| Health Canada | Elements of Real World Data/Evidence Quality throughout the Prescription Drug Product Lifecycle – March 2019 |
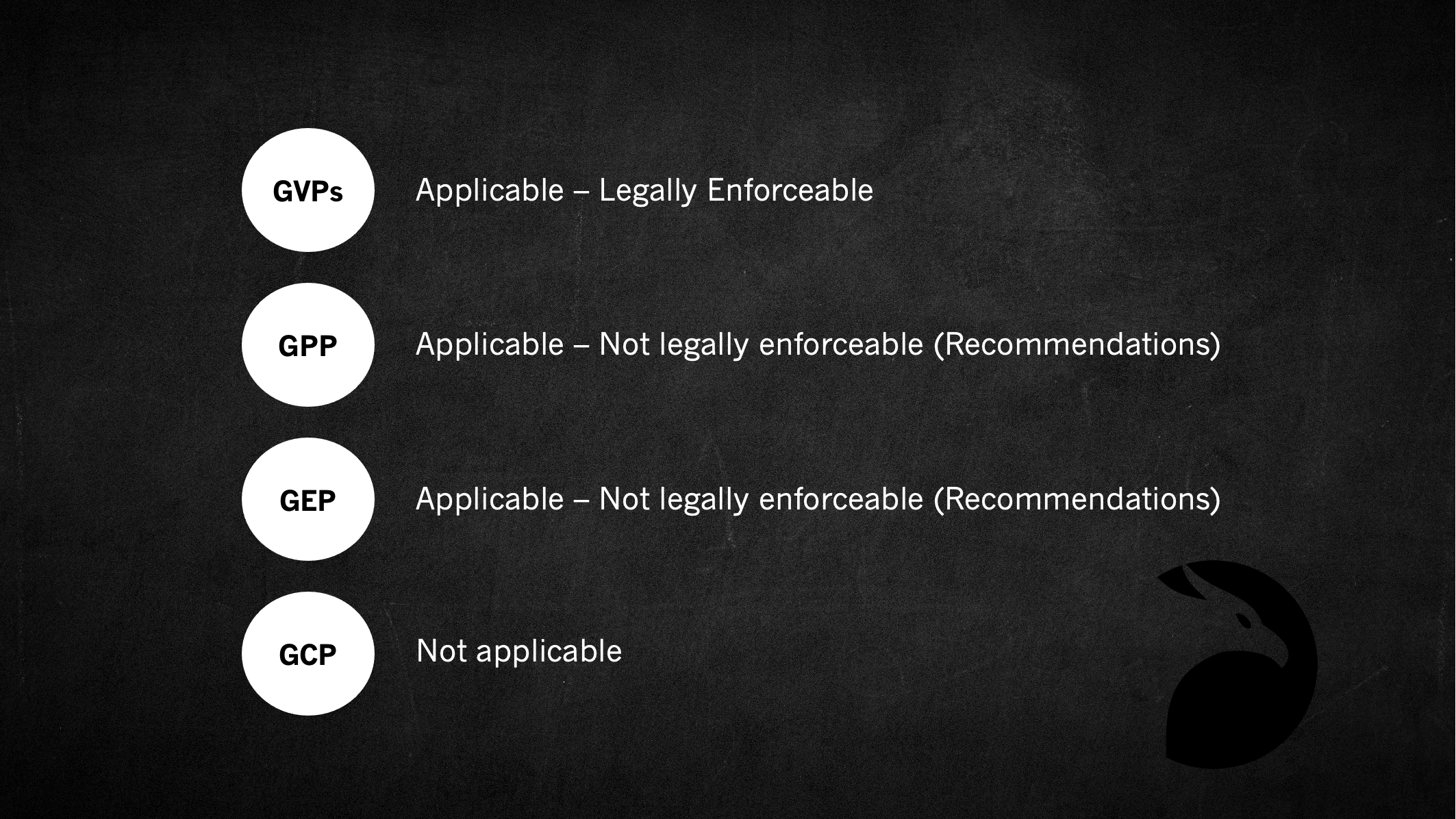
Which of the GxPs are Applicable to Non-Interventional Studies and Observational Studies?
The short answer is GVPs (EU only).
GVPs are derived from legislation and legally enforceable for non-interventional studies conducted in the EU (refer to Article 108a of Directive 2001/83/EC as amended) – See below…